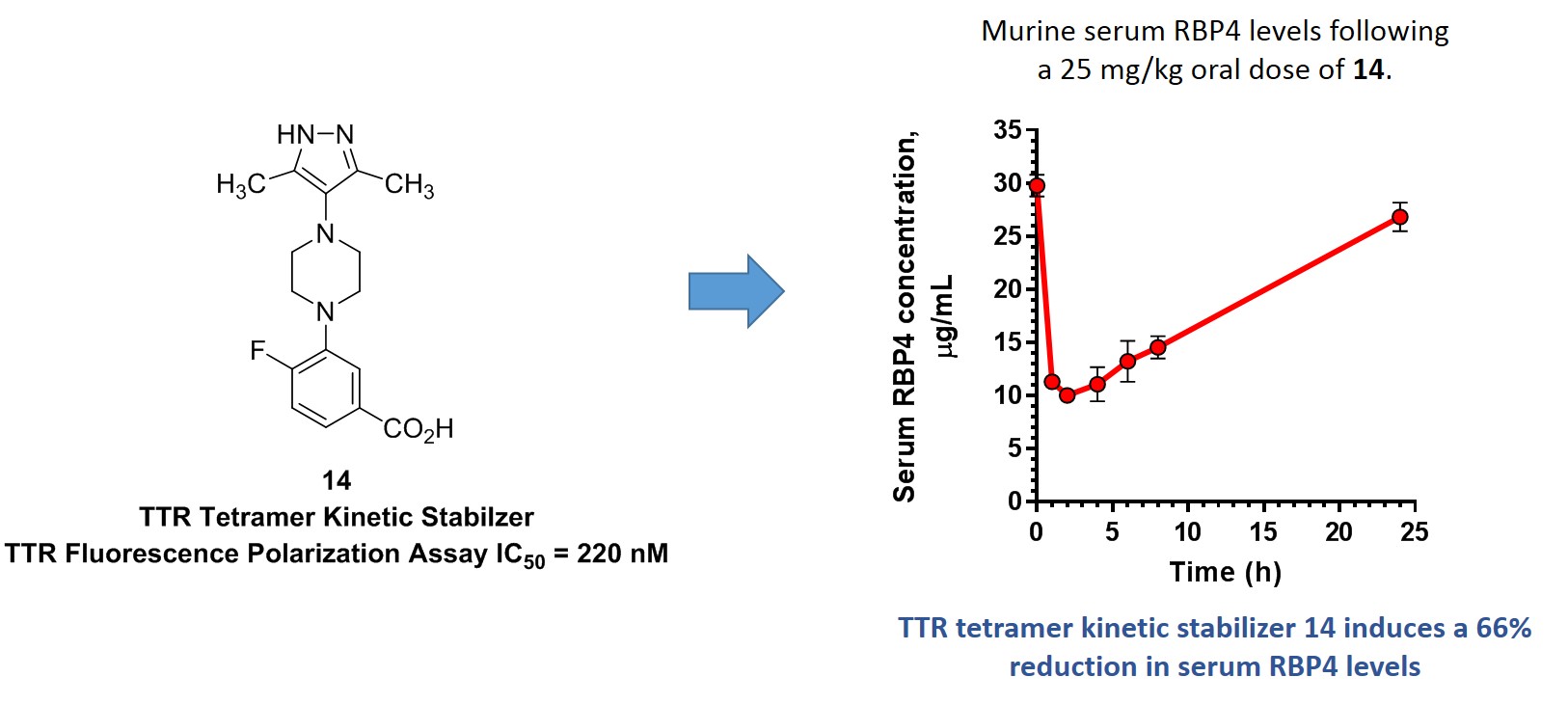
Dissociation of transthyretin (TTR) tetramers may lead to misfolding and aggregation of pro-amyloidogenic monomers, which underlies TTR amyloidosis (ATTR) pathophysiology. ATTR is a progressive disease resulting from the deposition of toxic fibrils in tissues that predominantly presents clinically as amyloid cardiomyopathy and peripheral polyneuropathy. Ligands that bind to and kinetically stabilize TTR tetramers prohibit their dissociation and may prevent ATTR onset. Drawing from clinically investigated AG10, we, together with our collaborator Dr. Konstantin Petrukhin (https://www.vagelos.columbia.edu/profile/konstantin-petrukhin-phd), designed a constrained congener (ACPHS-14) that exhibits excellent TTR tetramer binding potency, prevents TTR aggregation in a gel-based assay, and possesses desirable pharmacokinetics in mice. Additionally, ACPHS-14 significantly lowers murine serum retinol-binding protein 4 (RBP4) levels despite a lack of binding at that protein’s all-trans-retinol site. We hypothesize that kinetic stabilization of TTR tetramers via ACPHS-14 is allosterically hindering all-trans-retinol-dependent RBP4-TTR tertiary complex formation and that the compound could present ancillary therapeutic utility for indications treated with RBP4 antagonists, such as macular degeneration.
Funding: NIH 5R01EY028549, NIH 2R01EY028549-04A1
Cioffi, C. L.; Raja, A.; Muthuraman, P.; Jayaraman, A.; Jayakumar, S.; Racz, B.; Varadi, A.; Petrukhin, K. Identification of transthyretin tetramer kinetic stabilizers that are capable of inhibiting the retinol-dependent retinol binding protein 4-transthyretin interaction:Potential novel therapeutics for macular degeneration, transthyretin amyloidosis, andtheir common age-related comorbidities. J. Med. Chem. 2021, 64, 9010-9041; PMID: 34138572.
Licensing opportunity: https://inventions.techventures.columbia.edu/technologies/selective-ligands-of--CU20308