Ocular Diseases:
Stargardt Disease Type 1 (STGD1) is a rare inherited retinal disease (1 in 8,000 - 10,000 individuals) that afflicts approximately 30,000 individuals in the US. It is an autosomal recessive disease most commonly due to ABCA4 gene mutations and is a devastating neurodegenerative disease involving rapid growth of lesions (retinal geographic atrophy, or GA) in the central part of the retina known as the macula.
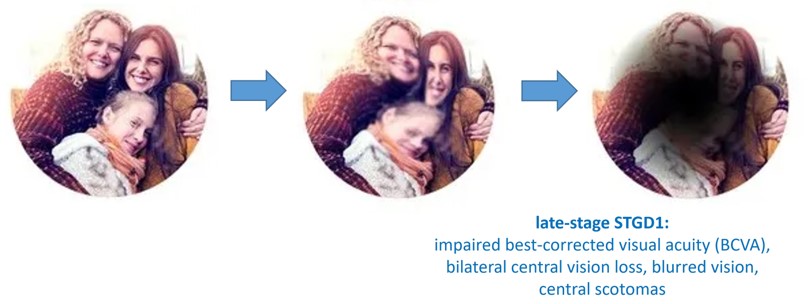
Early onset of the disease is highly aggressive and occurs in childhood/adolescence (7-18 years of age) with a subsequent peak in early adulthood, often correlating with a worse prognosis. Late onset is generally associated with a milder disease course. The disease involves a rapid decline in visual acuity (significantly impaired best-corrected visual acuity (BCVA)), blurred vision, and bilateral central vision loss with central scotomas.
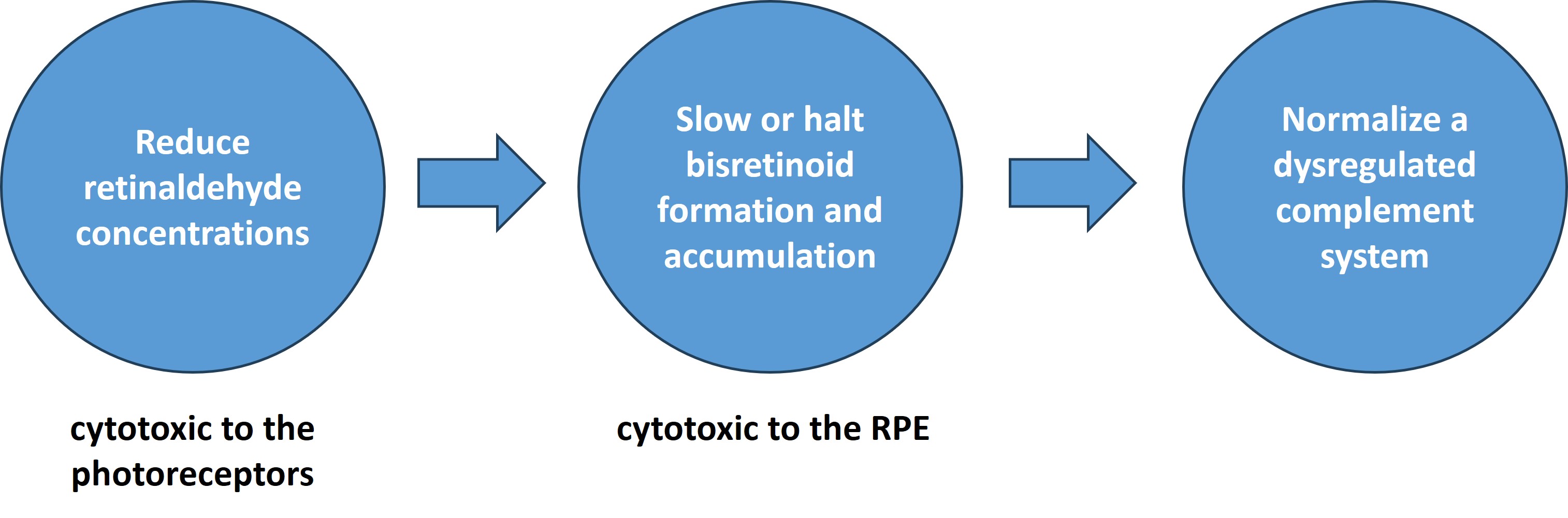
The pathophysiology of the disease is primarily driven by excessive accumulation of retinaldehydes and retinal bisretinoids. Inflammation in the retina via complement system dysregulation is also a major contributing factor in disease progression. Currently, there are no therapies available for STDG1 patients.
Atrophic age-related macular degeneration (dry AMD) is a slowly progressing neurodegenerative disorder in which rod and cone photoreceptors die in the the macula. It is the most prevalent form of AMD. AMD is the most common cause of legal blindness in individuals aged 60 years and older, and in the US, an estimated 15 million Americans suffer with dry AMD. Notably, the number of Americans afflicted with AMD is expected to double to 22 million by 2050 while the global patient population is expected to swell to 288 million by the year 2040.
Dry AMD is a multifactorial disease with multiple genetic correlations and environmental/lifestyle stressors contributing to disease incidence and pathophysiology. Underlying drivers of disease pathophysiology include inflammation/complement system dysregulation and excessive buildup of retinal bisretinoids. Two therapies have been recently approved for dry AMD treatment: Izervay and Syfovre. However, monthly or every other monthyl intravitreal injections are required. Thus, the discovery and development of novel and orally bioavailable drug therapies for dry AMD remains a critical unmet need.
Photoreceptor loss in dry AMD is secondary to abnormalities in the retinal pigment epithelium (RPE), a cellular layer that provides metabolic support to rods and cones. In dry AMD, age-dependent accumulation of lipofuscin in the RPE correlates with age-related increased incidences of the disease and represents one of several pathogenic factors contributing to the disease onset and progression. Bisretinoids, retinaldehyde dimers that are byproducts of the visual cycle, mediate lipofuscin toxicity and exert a myriad of deleterious effects on the RPE. Among them is the dysregulation of the complement system and inflammasome activation. Retinaldehyde toxicity may also play a role in the disease pathology.
Due to the fact that there appears to be a convergence on accumulation of bisretinoid-containing lipofuscin underlying STGD1 and, in part, dry AMD pathology, we are developing therapeutics that modulate the visual cycle and reduce the rate of formation and accumulation of cyctoxic bisretinoids via reducing excess pools of retinaldehydes. It has been hypothesized that pharmacological intervention in this manner may potentially slow or halt geographic atrophy (GA) lesion growth and prolong RPE and photoreceptor survival in the SDGT1 or dry AMD retina.
Active ocular disease projects:
TTR Tetramer Kinetic Stabilizers
Neuropathic Pain:
Approximately 116 million adults in the US suffer from chronic pain. An estimated 30-40% of these patients experience neuropathic pain. These symptoms include pain induced by non-painful stimuli (allodynia), increased sensitivity to pain (hyperalgesia), and other ongoing pain symptoms. Many neuropathic patients also suffer from comorbidities that reduce quality of life, such as depression, anxiety, or poor sleep quality. Existing pharmacological first-line treatments include gabapentinoids, tricyclic antidepressants, and selective serotonin-norepinephrine reuptake inhibitors, with second- and third-line treatments including opioids. These agents are limited as they provide moderate efficacy, induce dose-limiting side effects, or present a significant risk of tolerance, addiction, abuse, and lethality. Indeed, the dearth of effective therapeutics has led to an overreliance on opioid medications, which has fueled the escalation of opioid use disorder (OUD) incidence, morbidity, and mortality. Furthermore, overuse can also result in opioid-induced hyperalgesia. Thus, the development of new and effective chronic pain therapeutics devoid of the liabilities associated with opioid analgesics remains a highly critical and unmet need. Recent insights into the physiological adaptations underlying chronic pain have encouraged efforts to discover new classes of pain-relieving drugs targeting mechanisms that circumvent mu-opioid receptor engagement and circumvent OUD. We are exploring the therapeutic potential of glycine transporter 2 inhibitors for the potential treatment of neuropathic pain.
Active neuropathic pain projects:
Glycine Transporter 2 Inhibitors - Potential Analgesics for Neuropathic Pain
COVID-19:
Coronavirus disease 19 (COVID-19) is caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV2). The COVD-19 pandemic has had a devastating impact worldwide with an estimated 700 million cases and 7 million deaths since 2019. The SARS-CoV2 genome contains overlapping open reading frames encoding polyproteins that are cleaved via two viral proteases: papain-like protease (PLpro) and 3C-like protease (3CLpro).
Targeting the papain-like protease (PLpro) of SARS-CoV2 is an attractive drug discovery strategy because this viral cysteine protease is essential for coronavirus replication and pathogenesis. PLpro cleaves the viral polyprotein at specific sites to generate functional nonstructural proteins required for assembly of the replication–transcription complex. In addition to its role in viral maturation, PLpro also possesses deubiquitinase and deISGylase activities, which suppress host innate immune responses by removing ubiquitin and ISG15 modifications from host signaling proteins. Inhibiting PLpro therefore simultaneously disrupts viral replication and restores antiviral immune signaling, offering a dual mechanism of therapeutic benefit.
From a translational perspective, PLpro is also a tractable target for small-molecule inhibition, as its well-defined catalytic cysteine and substrate-binding cleft allow for structure-based drug design. High-resolution X-ray crystal structures are available, enabling rational inhibitor optimization. Importantly, PLpro differs structurally and functionally from human proteases, reducing the risk of off-target toxicity. Given the ongoing emergence of SARS-CoV-2 variants and the broader threat of future coronaviruses, PLpro inhibitors not only represent a potential treatment for COVID-19 but also provide a foundation for pan-coronavirus antiviral therapeutics.
Active COVID-19 projects:
Novel Antiviral Drugs – COVID-19