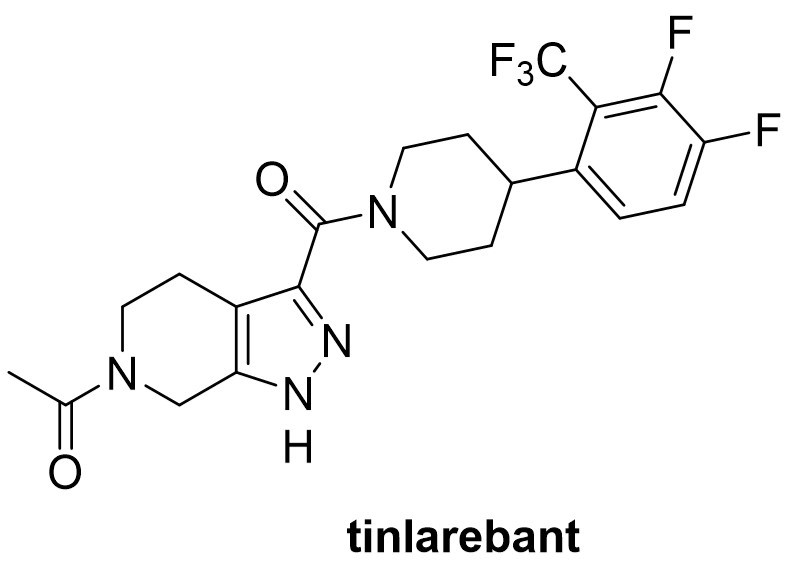
Accumulation of cytotoxic lipofuscin bisretinoids may contribute to atrophic age-related macular degeneration (AMD) pathogenesis, which is the most prevalent form of AMD. Retinal bisretinoid synthesis depends on the influx of serum all-trans-retinol delivered via a tertiary RBP4−TTR−retinol complex. During his tenure at Curia Global, Dr. Cioffi designed and is a co-inventor of the selective RBP4 antagonists including tinlarebant, which was licensed by Columbia University to Belite Bio (https://belitebio.com/). Dr. Cioffi served as the medicinal chemistry team project leader at Curia during the drug discovery phase of the project and collaborated with the Principal Investigator, Dr. Konstantin Petrukhin (https://www.vagelos.columbia.edu/profile/konstantin-petrukhin-phd) at Columbia University and the NIH Blueprint for Neurotherapeutics Network (https://neuroscienceblueprint.nih.gov/neurotherapeutics/bpn-small-molecules) Lead Development Team. Tinlarebant is currently in Phase 3 clinical trials for STGD1 and dry AMD (https://www.clinicaltrials.gov/search?intr=tinlarebant).
Funding: NIH U01NS074476 (Petrukhin)
Clinical Trials: DRAGON I, NCT05244304; DRAGON II, NCT06388083; PHOENIX, NCT05949593